Through the Anthony Nolan Cell & Gene Therapy Service’s provision of cellular material to cell and gene therapy researchers, we are familiar with industry standards and clients’ requirements. But we wanted to find out more about what drives decision making when selecting a cell source provider, and set out the key questions you should be asking.
So we conducted a poll with leaders in the cell and gene therapy industry to find out what mattered to them…

Quality and availability
The most common challenges identified in obtaining cell material were quality and availability. In particular, the majority of respondents (77%) said that access to high-quality cell sources would actually expedite their developments.
The opportunity to accelerate R&D is of crucial importance in the cell and gene therapy space, where progress is rapid and there are increasing numbers of organisations competing.
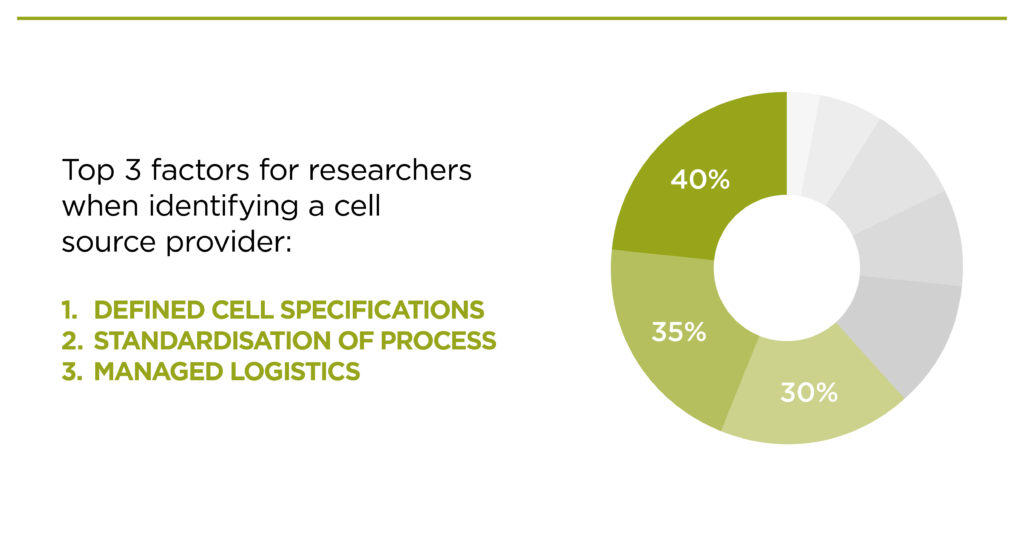
Bespoke materials
When asked what factors are important when identifying a cell source provider, the most common answers were:
- Defined cell specifications
- Standardisation of process
Managed logistics
This tells us that clients feel that getting bespoke material provided to specification is of the utmost importance. In contrast, only 5% said that availability/access to off-the-shelf frozen material was of interest.
Researchers also selected location as important i.e. whether the material is locally sourced, as well as price.
Ethically sourced
15% of respondents said that a deciding factor is whether material was ethically-sourced. This is a unique consideration for organisations working in cell and gene therapy, compared with traditional pharma manufacturing. As the cell and gene therapy space has become more established, we’ve seen more clients scrutinising ethics to ensure that the effort of the donor is proportionate to the potential patient benefit.
Asking the right questions
A cell source provider should meet all of the above requirements, without having to consider a trade-off e.g. between ethics and price.
That’s why it’s really important to ask the right questions to help you identify a partner that meets your needs:
- How do you procure material? Do you use any third parties?
- How can you provide material to my specifications?
- Are donors paid for donations?
- Are appropriate ethics considerations in place? Does this meet regulations in the country where the material will be delivered?
- What processes are in place to ensure that cells are ethically sourced?
- Will I have full visibility on the donor consent?
- Do you have experience in providing material to researchers?
- What are the long-term implications of working together - from early stage research through to clinical application?
Ultimately, we believe that you need to find a partner that is aligned exactly to your core mission: to improve the lives of patients through the development of cell and gene therapies.
